Abstract: Anionic surfactant English chemical term: An-ionic surfactant. A type of surfactant. After dissociation in water, hydrophobic anions are generated. For example, fatty alcohol sodium sulfate is dissociated into two parts of ROSO2-O- and Na, surrounded by water molecules. The negatively charged ROSO2-O- has surface activity. 1. Now the national standard uses chloroform (chemically pure or analytically pure) extraction, methylene blue colorimetric detection principle, there are two main problems: 1. The amount of reagent is too large and extraction is very inconvenient. Easy to leak, causing errors 2. The lower detection limit cannot meet the needs of surface water, rivers, lakes, seawater and drinking water, and can only be suitable for water bodies with certain pollution. The method we provide is molecular fluorescence photometry, which has the following advantages of Ming County: 1. The reagent needs a small amount, the extraction is simple, and it can be basically completed in two minutes. 2. Acridine yellow is used as a fluorescent staining agent, that is, fluorescent tracer, the cost is very low, and it is easy for most laboratories to have this condition. 3. Low detection limit, detection range: 0.025 to 2.0mg / l, high concentration can be diluted. Therefore, there is a good possibility to replace the current national drinking water standards in China. Hope to arouse the attention of the relevant national standard-setting bodies. 4. Can analyze natural water, sewage, drinking water, well water, sea water. To meet the monitoring of the concentration of detergent active ingredients in different industries. 5. This method considers the potential interference of aromatic hydrocarbon-containing petroleum, which can be reduced by n-hexane extraction. 6. Finally, after practical experience in the South China Environmental Research Institute of the Ministry of Environmental Protection of China, the standard curve can reach four nines, and the measurement is stable and reliable. Cationic surfactant Reagent 50g / L sodium hydroxide solution 5% hydrochloric acid solution 100mg / L eosin solution 100mg / L cationic surfactant solution Borate buffer 20.0mg / L cationic surfactant solution 1.0mg / L cationic surfactant solution Acetone, analytically pure Chloroform, chemically pure or analytically pure 5% sodium hydroxide solution Anionic surfactant 0.1mol / L hydrochloric acid solution 100mg / L acridine yellow solution 100mg / L anionic surfactant solution 10.0mg / L anionic surfactant solution 1.0mg / L anionic surfactant solution Chloroform, chemically pure or analytically pure During calibration, the blank solution was poured into a separatory funnel into 5ml of distilled water, 2ml of borate buffer solution was added, 0.5ml of eosin solution and 5ml of chloroform were added, extraction was performed for 1min. , Mix 1ml of acetone, pour the solution into the sample cell to measure the fluorescence signal. Pour 9ml of distilled water into the separatory funnel, add 1ml of acridine yellow solution, 1ml of hydrochloric acid solution, 5ml of chloroform, extract for 1min, after layering, use the bottom layer to measure the fluorescence signal. The calibration solution with a certain concentration changes 5ml of distilled water in the blank solution step to a standard solution with a certain concentration, and the remaining steps are the same. Pour 5ml of standard solution of certain concentration, 4ml of distilled water, add 1ml of acridine yellow solution, 1ml of hydrochloric acid solution, 5ml of chloroform, and extract for 1min. After layering, use the bottom layer to measure the fluorescence signal. Measure the extraction of the oil product in the first step: add 5 ml of sample to the separatory funnel, (the expected cationic surfactant concentration is not less than 0.05 mg / l), and add 10 ml of hexane. The extraction was performed for 1 minute, and the hexane layer was discarded after the separation. If the expected cationic surfactant concentration is less than 0.1 mg / l, increase the water sample volume to 20 cm3. The second step: add 5ml of the sample prepared in the first step, 2ml of borate buffer, 0.5ml of eosin solution and 5ml of chloroform to the separatory funnel, take 1min, after layering, take 4ml of the bottom layer to the probe with a stopper Mix 1ml of acetone in the tube and pour the solution into the sample cell for measurement. The first step of product extraction: Only when analyzing some highly contaminated samples containing oil products, whose concentration is higher than 5mg / l and the analyzed sample produces chloroform extract (no dye). Method: Put 5ml sample into 50ml separatory funnel, add 4-5ml distilled water and 10ml hexane. The mixture was stirred for 30 sec, the layers were settled, and the ethane layer was separated. Step 2: Pour 5ml of the sample prepared in the first step, 4ml of distilled water, 1ml of acridine yellow solution, 1ml of hydrochloric acid solution, and 5ml of chloroform into the separatory funnel, and extract for 1min. After layer separation, use the bottom layer to measure. Method for detecting anionic surfactant concentration in natural water, drinking water and wastewater samples using "HIGHSKY-02" fluorescent multi-parameter liquid analyzer Beijing China 2010 1 Scope and use This article introduces a method for measuring the anionic surfactant concentration of natural water, drinking water and wastewater samples using the "HIGHSKY02" liquid analyzer using fluorescence technology. The concentration suitable for this method is 0.025 to 2.0 mg / l (no sampling or extraction dilution). If the concentration of the anionic surfactant in the analyzed sample is higher than the measurable concentration range, the sample is allowed to be diluted as follows: the sample concentration is diluted into the measurable concentration range and the dilution ratio is not higher than 100 times. How to eliminate the interference caused by the coloration of petroleum products and organic impurities in the extract? This technology will be introduced in Section 7.1. 2 uncertainty The uncertainty of the anionic surfactant measurement method introduced in this article is not higher than the value in Table 1. Table 1 Uncertainty of measurement when confidence Ð = 0.95 Range, mg / l measurement uncertainty, ??,% From 0.01 to 0.1 (including 0.1) From greater than 0.1 to 1.0 (including 1.0) From greater than 1.0 to 2.0 (including 2.0) 50 30 20 When the sample is diluted with distilled water, the uncertainty is equal to the uncertainty of the anionic surfactant measurement of the diluted sample provided. The dilution uncertainty of the sample should not exceed 3%. 3 Appliances, reagents and materials The following utensils, reagents, materials and solutions are used for the determination of anionic surfactant concentration. 3.1 Appliances and their standards "HIGHSKY02" liquid analyzer General laboratory balance with second-class accuracy
FAQ
Q1: Are you manufacturer or trading company?
A1: We are manufacturer.
Q2: What is the material of products?
A2: Natural Birch.
Q5: Do you provide samples?
A5:Yes, free samples available.
Wooden Tableware Spoon Tableware Set,Disposable Spoon,Disposable Spoon Set,Disposable Wood Spoon Dalian Yongtailong Wood Industry Co.,Ltd , https://www.ytldisposablegoods.com


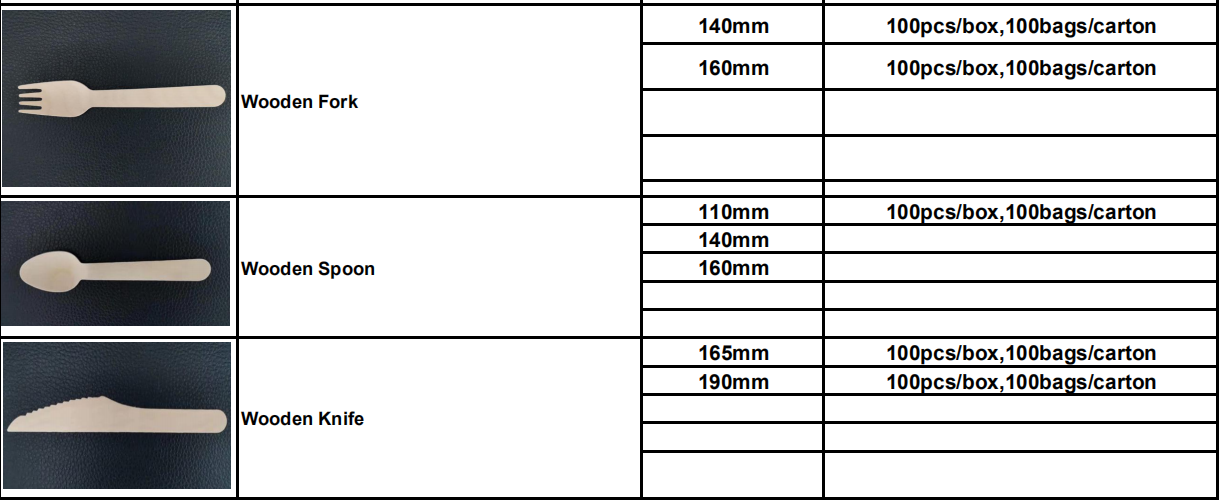
Q3: How long will you deliver the products?
A3: 30~60 days after receiving 30% T/T deposit.
Q4: What is the payment term?
A4: T/T 30% as deposit in advance and balance 70% should be paid when goods ready to ship or L/C at sight.
November 08, 2020